we connect people and industry
Latest news and topics
In this section you will find a social media wall from Walls.io. In order to display this social media wall, you must agree to the use of external content (incl. cookies). By activating the "Load content" button, you agree to external content being displayed to you. This may transmit personal data, such as IP address and cookie information, to third-party platforms. Information can be found in the privacy policy and the setting can be changed again at any time in the cookie settings.
We manage and operate CHEMPARK
Education & Training
Education and training for individuals or whole companies? We do both. Choose directly from our range of seminars - or talk to us to find a solution for your company.Waste Disposal
We dispose of residual materials from chemical production in a sustainable manner. Recycling, landfilling, incineration - all this is part of our spectrum. We advise companies on disposal concepts and take care of the implementation.
Settlement
You want to become a CHEMPARK partner? Talk to us. We would be pleased to present our range of services to you in detail.
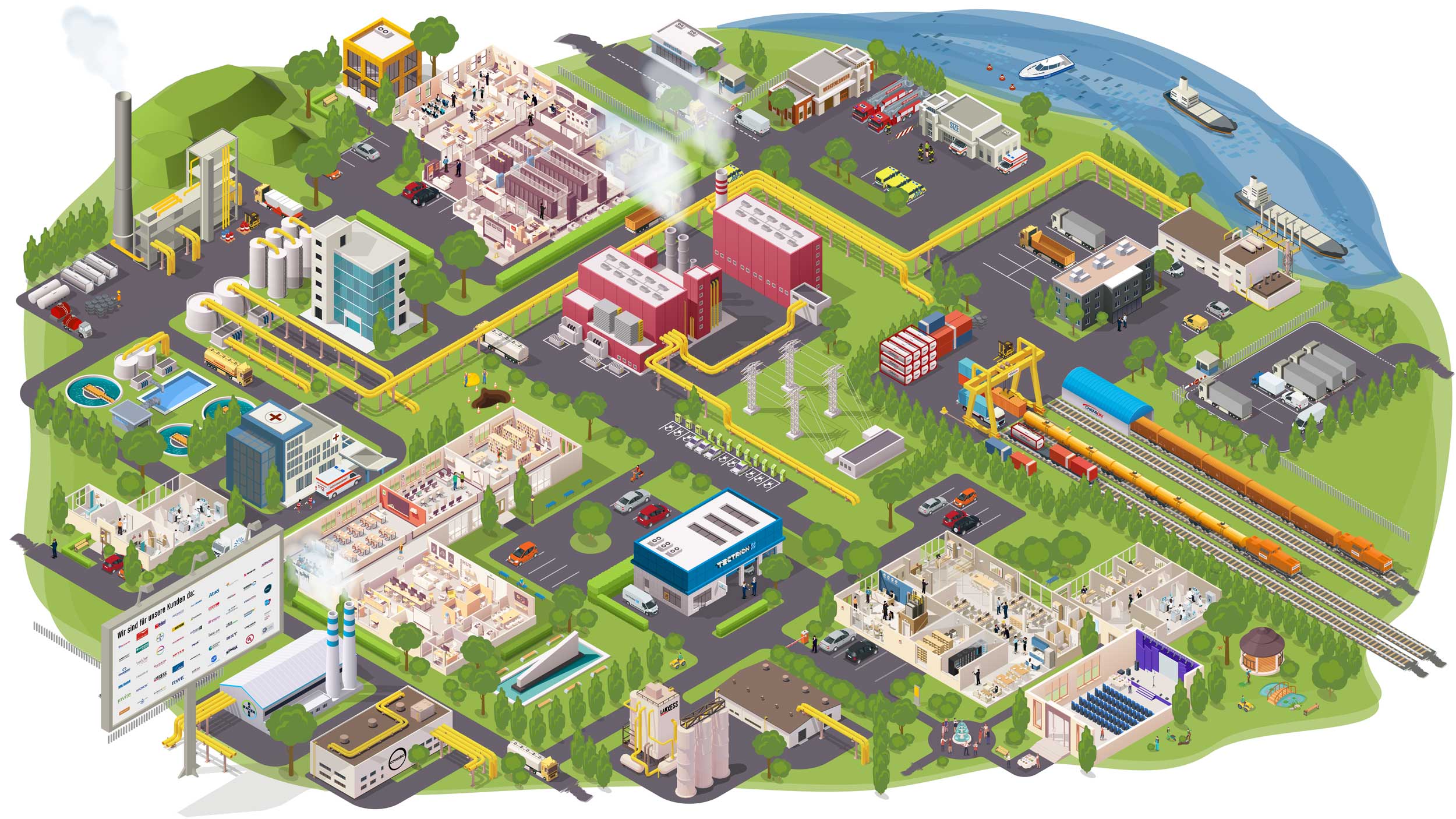
Supply
Steam, gas, electricity, cooling, heat, water: we have it all ready. Via our own networks and pipelines, we deliver it directly to the customer on site, taking care of safe operation and reliable delivery.
Electromobility
Charging stations for renewable energies: At the CHEMPARK and beyond, we connect your vehicles to the grid - and your company ahead.
Safety and Security
Fire department, rescue service, plant safety, environmental monitoring: We ensure safety at the site. In prevention and acute cases of any dimension. To achieve this, we have the latest technology, highly qualified personnel and practiced processes at our disposal.
Infrastructure
Industry needs reliable infrastructure. Therefore, we take care, for example, of the planning, construction, maintenance, dismantling, provision and operation of site roads, bridges and tunnels, the quay and port infrastructure, the CHEMPARK rail network and stationary and mobile flood protection facilities.
Logistics (Chemion)
Rail, truck or ship: Chemion Logistik offers a wide range of logistics services in the chemical and chemical-related industries. Many years of experience in site logistics and in the handling of hazardous goods characterize us.
Projects & events
From small events to annual general meetings: Our event service provides support for small and large projects at the site and in the surrounding area.
Analytics
We offer a comprehensive analytical service for research, development and production in industry. Our qualified analytical experts are at your side as dedicated and reliable partners with many years of experience, in-depth expertise and extensive product experience.
Consulting and expertise
We have the experts for all relevant topics in the chemical industry. Professional analyses and expert opinions are an important basis for business decisions. We supply those.
Engineering (Tectrion)
Repairs, service, maintenance: Tectrion's team knows exactly the pulse of the plants. They are the partner for large and small orders directly on site.
Education & Training
Waste Disposal
Settlement
Supply
Electromobility
Safety and Security
Infrastructure
Logistics (Chemion)
Rail, truck or ship: Chemion Logistik offers a wide range of logistics services in the chemical and chemical-related industries. Many years of experience in site logistics and in the handling of hazardous goods characterize us.
Projects & events
Analytics
Consulting and expertise
Engineering (Tectrion)
Repairs, service, maintenance: Tectrion’s team knows exactly the pulse of the plants. They are the partner for large and small orders directly on site.
For renewed chemistry: With 3 principles towards sustainability
We are CURRENTA. We manage and operate CHEMPARK – one of the largest chemical sites in Europe located in Leverkusen, Dormagen and Krefeld-Uerdingen. As a modern service company, we put our customers first – by creating best conditions for their research and production facilities. Our range of services includes e.g. on-site material and energy supply, a wide variety of analytical services, environmental issues management, infrastructure services and reliable safety concepts. Everything our customers need. For new chemistry.
Real transformation: Change or disappear
Investments in future technologies to secure the location
An opportunity that we can only master in collaboration
#understand #change #improve
For us, safety has top priority. This self-image is the basis of our work, our actions, our processes. We regularly review our safety standards and continuously improve. The exchange with you is important to us. Use our dialogue formats, we look forward to getting in touch with you.

Tim Hartmann
Chairman of the Management Board

Hans Gennen
Member of the Management Board

Wolfgang Homey
Member of the Management Board
Analytics
CURRENTA offers a comprehensive analytical service for research, development and production in industry. Our qualified analytical experts are at your side as dedicated and reliable partners with many years of experience, in-depth expertise and extensive product experience. Service analytics for industry is not limited to the measurement of analytical parameters.